Medical Device Suppliers Challenges
Today, medical device suppliers are faced with numerous challenges such as:
- lack of usage of a consistent master data exchange format (sometimes usage of word documents, excel, papers, …)
- suboptimal efficiencies, as customers don’t currently synchronize master data with their suppliers, across borders or within their regions
- high level of resources needed to upload and maintain product catalog
- lack of unambiguous identification of physical or legal locations involved in transactions In looking to address these issues,
Medical device suppliers wanted to ensure that the process was efficient, robust, and comprehensive to meet customer needs for high-quality data. This would ultimately improve patient and clinician safety and lower healthcare costs.
At the same time, suppliers acknowledged the value of using GS1 Standards to identify products within their supply chain to overcome these challenges and meet their customer’s requirements.
Access to accurate and current product information is paramount in the healthcare industry, where even the smallest details can have significant implications for patient safety and treatment outcomes. From life-saving pacemakers to intricate surgical screws, healthcare professionals rely on up-to-date data to ensure the safe and effective use of medical devices.
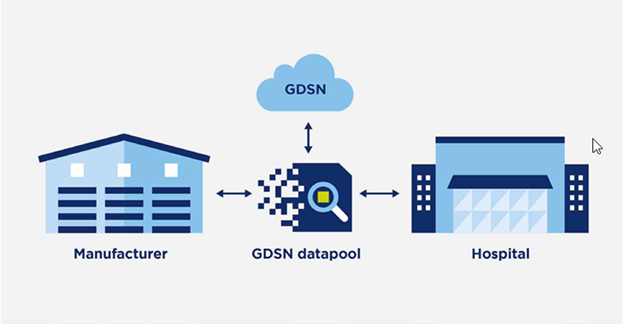
This critical information exchange is facilitated through the Global Data Synchronization Network (GDSN) provided by GS1. By leveraging GDSN, healthcare stakeholders can access standardized and synchronized product data, enhancing transparency and reliability throughout the supply chain. The adoption of GDSN underscores a commitment to patient safety and quality care. With access to accurate product information, healthcare providers can make informed decisions, mitigate risks, and optimize patient outcomes.

Understanding GDSN: A Game-Changer for Medical Device Suppliers
The Global Data Synchronization Network (GDSN) is a worldwide network that enables the standardized exchange of product information between trading partners in real time. It provides a centralized platform for the synchronization of product data, ensuring consistency and accuracy across the supply chain. For medical device suppliers, leveraging GDSN offers a host of benefits, including:
Standardized Product Information: GDSN ensures that product data is standardized according to global GS1 standards, eliminating discrepancies and improving data quality.
Real-Time Data Synchronization: With GDSN, product information is synchronized in real-time, allowing suppliers to access the most up-to-date data at any given time.
Global Reach: GDSN facilitates the exchange of product data across international borders, enabling suppliers to manage their global product portfolios more effectively.
Regulatory Compliance: By adhering to GS1 standards, medical device suppliers can ensure compliance with regulatory requirements in various markets, minimizing the risk of non-compliance penalties.
Enhanced Efficiency: GDSN automates data exchange processes, reducing manual effort and minimizing the risk of errors associated with manual data entry.
How Medical Device Suppliers Utilize GDSN for Global Product Master Data Management
Data Standardization: Medical device suppliers ensure that product information is standardized according to GS1 standards, including Global Trade Item Numbers (GTINs), Global Location Numbers (GLNs), and product attributes.
Data Publication: Suppliers publish their product data to the GDSN, where it is synchronized with trading partners in real time. This ensures that all stakeholders have access to accurate and consistent product information.
Regulatory Compliance: Suppliers use GDSN to ensure compliance with regulatory requirements in different markets by providing the necessary product data in a standardized format.
Data Governance: Suppliers establish robust data governance processes to maintain data integrity and accuracy within the GDSN, including data validation, verification, and monitoring.
Continuous Improvement: Suppliers continually review and update their product data in the GDSN to reflect changes such as new product launches, updates, or discontinuations, ensuring that stakeholders have access to the latest information.
Conclusion
GDSN has revolutionized the way medical device suppliers manage their global product master data, providing a centralized platform for standardized data exchange and ensuring compliance with regulatory requirements. By leveraging GDSN, suppliers can streamline their operations, enhance collaboration with trading partners, and ultimately deliver safer and more effective medical devices to patients worldwide.
Commport GDSN Solution for Healthcare
Download: GDSN Buyers Guide
Empower your business with global data synchronization; download our GDSN Buyer's Guide today and take the first step towards streamlined, accurate, and compliant product data management.
Frequently Asked Questions
GDSN employs GS1 standards to standardize product data, ensuring consistency across the supply chain. Real-time synchronization also minimizes data discrepancies
Yes, GDSN enables suppliers to provide product data in a standardized format, facilitating compliance with regulatory requirements in different markets.
GDSN enhances collaboration and transparency among trading partners by providing access to standardized and up-to-date product information in real-time.
Suppliers should update their product data in GDSN regularly to reflect changes such as new product launches, updates, or discontinuations, ensuring stakeholders have access to the latest information.
Suppliers can establish robust data governance processes, including data validation, verification, and monitoring, to maintain data integrity and accuracy within GDSN.




